Introduction
TP53-mutated acute myeloid leukemia (AML) carries a dismal prognosis. Optimal induction therapy is uncertain, and it remains unclear whether allogeneic stem cell transplant (allo-SCT) improves survival.
Methods
We investigated 89 cases of TP53-mutated AML diagnosed at or referred to Thomas Jefferson Hospital between April 2016 and May 2023 and reviewed clinical characteristics and outcomes. Remission status was determined by bone marrow biopsy and aspiration after at least 1 cycle of anti-leukemic therapy (ALT). ALT was classified into high- or low-intensity treatment. High-intensity treatment was defined as regimens containing high-dose cytarabine (≥ 200 mg/m 2/day) for at least five consecutive days in combination with an anthracycline. Low-intensity treatment was defined as hypomethylating agent (HMA)-based therapy containing either azacitidine or decitabine with or without venetoclax.
Results
Median age at diagnosis was 66 (range, 23-91). Forty-nine percent had de novo AML, and the rest had secondary AML. Ninety-one percent had newly diagnosed AML and 9% were found to have a TP53 mutation at relapse. Median somatic mutation count as detected by next-generation sequencing (NGS) was 2 (range, 1-9). Thirty percent were treated with high-intensity treatment, 46% with low-intensity treatment, and 22% received supportive care only. Detailed patient characteristics are in Table 1.
Median overall survival (OS) for the entire group was 3.17 months [95% confidence interval (CI) 1.90-5.13 months], and 5.13 months (95% CI 3.83-8.00 months) for patients who received any ALT. Of 69 patients who received ALT, composite complete remission (CRc) rates were 26% with high-intensity treatment and 34% with low-intensity treatment ( p = 0.56). Fifty-six percent did not achieve a remission, and 12% were not able to be assessed due to early mortality. The 30-day mortality was 15% and 7% with high- and low-intensity treatments, respectively. OS was not different between patients who received high- and low-intensity treatments (5.13 months vs 5.27 months, p = 0.65).
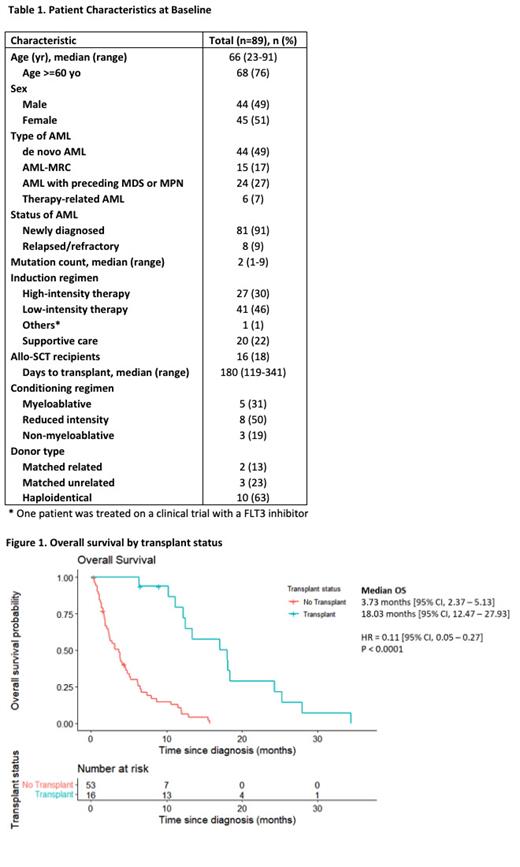
Twenty-four percent underwent allo-SCT with a median follow-up of 15.2 months. The median time to transplant was 180 days from time of diagnosis (range, 119-341). Median hematopoietic cell transplantation-specific comorbidity index was 3 (range, 1-7). The majority of patients (69%) received a reduced intensity or non-myeloablative regimen, and 63% received a haploidentical transplant. Eighty-one percent of transplant recipients were in CR or CR with incomplete count recovery at the time of transplant; the remaining had either primary induction failure or relapsed disease. Median OS was significantly longer for patients who received allo-SCT compared to those who received chemotherapy only (18.0 months vs 3.7 months, p <0.0001) (Figure 1). Cumulative incidence of relapse post-transplant was 36% at 1 year and 79% at 2 years.
Conclusions
TP53-mutated AML is associated with a dismal outcome. In our single-center analysis, approximately one-third of patients achieved remission after initial ALT. Consolidation with allo-SCT is associated with improved survival, however relapse risk remains high. Treatment strategies aimed at reducing disease relapse post-transplant are warranted.
Disclosures
Kasner:BMS: Research Funding; Kartos/Telios: Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Kronos: Membership on an entity's Board of Directors or advisory committees; Gilead: Research Funding; Pfizer: Research Funding. Keiffer:Cyteir Therapeutics: Research Funding; Prelude Therapeutics: Research Funding; Astellas: Membership on an entity's Board of Directors or advisory committees; Abbvie: Research Funding. Wilde:Gilead: Research Funding. Gergis:Iovance: Current equity holder in publicly-traded company; Gamida: Consultancy, Current equity holder in publicly-traded company; Kite, a Gilead Company: Honoraria, Other: Travel Support and other relationship, Speakers Bureau; Astellas: Speakers Bureau; Jazz: Consultancy, Honoraria, Other: Travel Support, Speakers Bureau; Incyte: Honoraria, Other: Travel Support, Speakers Bureau; Novartis: Honoraria; Thomas Jefferson University: Current Employment.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal